Research
Preliminary Work
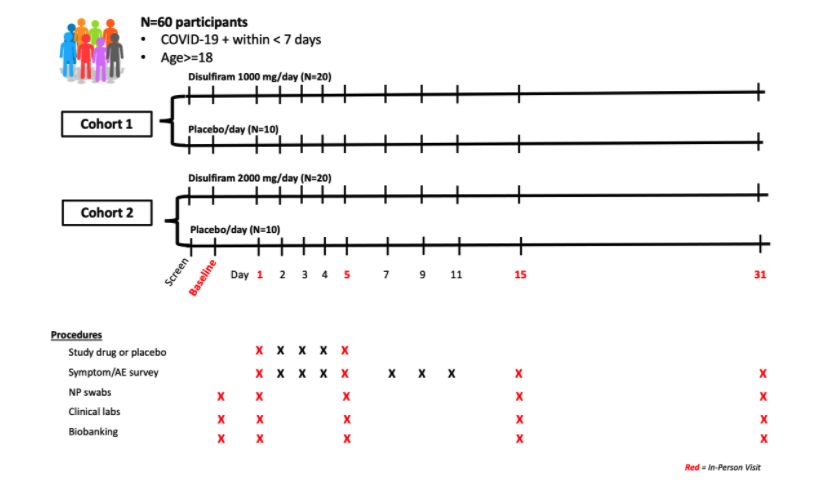
Disulfiram for COVID-19 (DISCO) trial (Phase 2 Trial)
The identification of a safe, effective treatment for individuals with early symptomatic COVID-19 that prevents progression to more severe disease would have immediate public health implications. A hallmark of severe COVID-19 disease is immune system dysregulation called "cytokine storm." Multiple studies have reported that patients with severe disease demonstrate elevated levels of pro-inflammatory cytokines early in disease, such as interleukin (IL)-6. Recent data suggests multiple mechanisms by which disulfiram, an FDA-approved drug for the treatment of alcohol dependence disorder with a good safety profile and easy dosing schedule, may act on COVID-19 both as a direct antiviral as well as an indirect anti-inflammatory agent (e.g., via inhibition of the NLRP3 inflammasome and cytokine production). We have previously shown that disulfiram, given at high doses (2000 mg/day), is well-tolerated in HIV-infected individuals. Disulfiram has a short half-life ~7.5 hours with >90% of drug eliminated within 3 days post-dose, allowing quick reversal of any potential adverse effects. For the DISCO trial, recent COVID-19+ individuals will be randomized (2:1) to receive either oral disulfiram (1000 mg/day or 2000 mg/day) for 5 consecutive days or placebo to assess the effects of the drug on COVID-19 symptom severity, SARS-CoV-2 viral shedding, and inflammation.
COVID-19 Host Immune Response Pathogenesis (CHIRP) Cohort Study
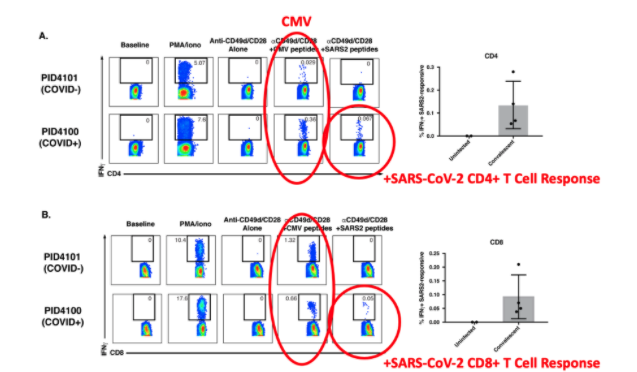
The COVID-19 pandemic has caused devastating morbidity and mortality worldwide. However, the virus itself does not seem to explain the person-to-person variability in clinical course (ranging from asymptomatic carriage to severe lethal respiratory disease, as well as long-term inflammatory sequelae). Variation in host genetics and host-specific immune responses likely play a major role in determining the severity of COVID-19. We are performing in-depth sampling of blood, sputum, nasal swab, saliva, urine, and stool to apply advanced single cell immunogenetic approaches to predict the specific immune cells and inflammatory pathways that determine severity of COVID-19. Our preliminary data from the CHIRP study demonstrates that convalescing COVID+ individuals mount robust T cell responses against SARS-CoV-2, suggesting an important role for T cells in viral clearance. These data will inform the safety of designing COVID clinical trials aimed at modulating these pathways.
The COVID-19 pandemic has caused devastating morbidity and mortality worldwide. However, the virus itself does not seem to explain the person-to-person variability in clinical course (ranging from asymptomatic carriage to severe lethal respiratory disease, as well as long-term inflammatory sequelae). Variation in host genetics and host-specific immune responses likely play a major role in determining the severity of COVID-19. We are performing in-depth sampling of blood, sputum, nasal swab, saliva, urine, and stool to apply advanced single cell immunogenetic approaches to predict the specific immune cells and inflammatory pathways that determine severity of COVID-19. Our preliminary data from the CHIRP study demonstrates that convalescing COVID+ individuals mount robust T cell responses against SARS-CoV-2, suggesting an important role for T cells in viral clearance. These data will inform the safety of designing COVID clinical trials aimed at modulating these pathways.
Publications
- SARS-CoV-2-specific T cells exhibit unique features characterized by robust helper function, lack of terminal differentiation, and high proliferative potential
- Protracted yet coordinated differentiation of long-lived SARS-CoV-2-specific CD8+ T cells during COVID-19 convalescence
- Novel RT-ddPCR assays for measuring the levels of subgenomic and genomic SARS-CoV-2 transcripts
- Host Variation in Interferon, MHC Class I, Glycosylation, and Viral Transcription Genes Predict HIV Persistence
Collaborative Science
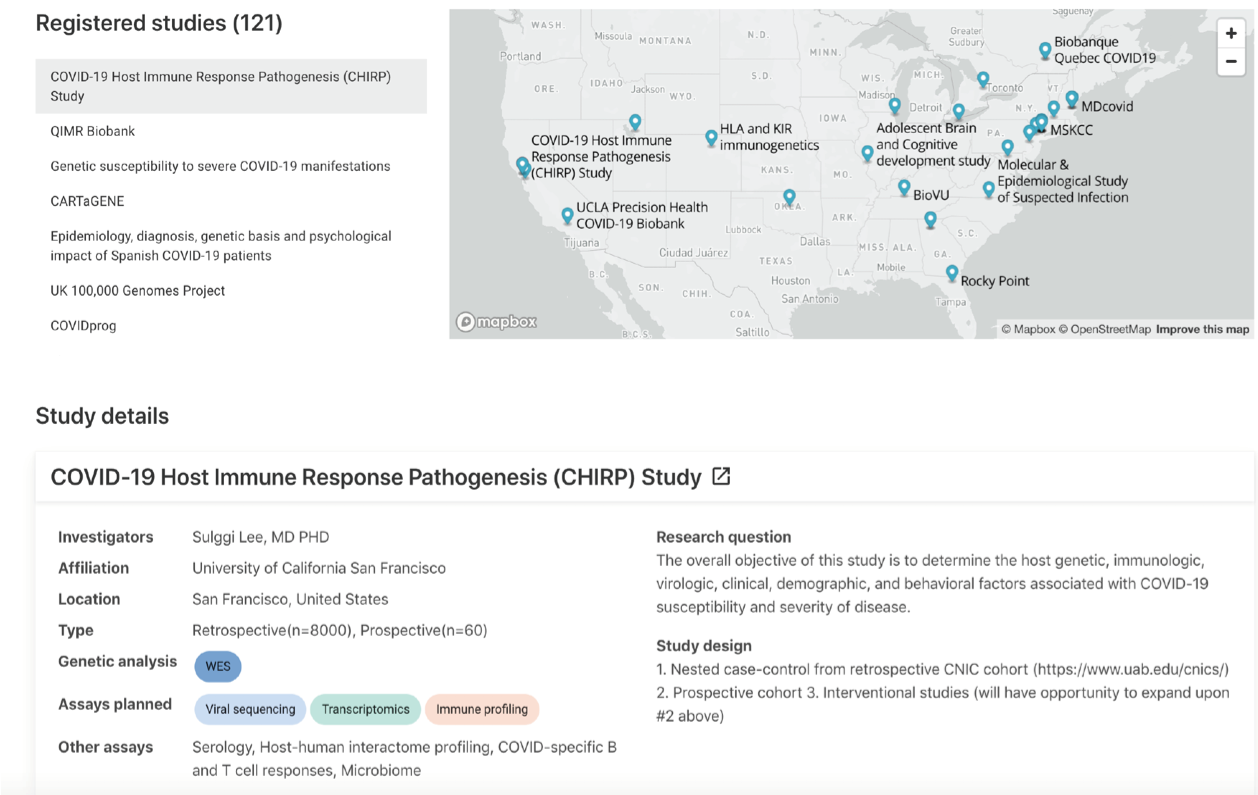
Partners




EMORY CHIRP Team: Eliver Ghosn, Astrid Kosters, Devon Eddins, Ben Babcock, Junkai Yang
Funding
We are currently seeking funding for our clinical research study. Please contact us if you are interested in contributing to our efforts.
*Please note on the giving form that the gift is in honor of Dr. Sulggi Lee to ensure that it is directed towards her research.
Donate Now
~$13,000 will approximately cover the cost to enroll ONE study participant to complete the study (material and supplies only; does not include research salary support costs). Supplies and materials included in these costs are blood collection tubes, nasal/oropharyngeal swabs, biospecimen collection cups, PPE, alcohol-based cleaning supplies, mobile van costs, sample processing, sample shipping, and biospecimen storage costs.
- Fast Grants Award
- Van Auken Private Foundation
- David Henke
- The Roddenberry Foundation, Gladstone Institutes
- Program for Breakthrough Biomedical Research (UCSF)
